Baby Milk Action press release 25 July 2017
The Natural History Museum is hosting an event on 25 July 2017 promoting Danone’s Aptamil follow-on formula to parents. The museum is facing criticism for undermining advice from NHS Choices to parents, which is clear that follow-on formula is an unnecessary product – babies who are not breastfed or receiving expressed breastmilk can be fed on infant formula to 12 months of age. Thereafter breastmilk or normal family milk can be given. See:
http://www.nhs.uk/Conditions/pregnancy-and-baby/Pages/types-of-infant-formula.aspx#followon
Update: Responding today to Baby Milk Action the Museum press office said:”Aptamil is not a partner of the Museum and their involvement with the event is through Time Out, who is delivering the event” (full comment below).
Update: Baby Milk Action asked the museum it has a policy in place regarding hosting such events, or will consider putting one in place and was told: “We hold a wide variety of commercial events and it is made clear to the third party host that doing so is not an endorsement of their product, service or views.” This does not address the fact that members of the public are associating the Aptamil promotion directly with the Museum. However, the Museum also told Baby Milk Action, “Thank you for highlighting the issue to us and the Museum will take this into consideration with future events.”
Advertising for the event organised in partnership with Time Out London also appears to break the Infant Formula and Follow-on Formula Regulations (2007) as it promotes the Aptamil brand and logo used for infant formula. Baby Milk Action is calling on Trading Standards to investigate.

Follow-on formula is marketed for use from 6 months of age and was introduced by baby feeding companies in an attempt to bypass restrictions on advertising and promoting infant formula for use from birth. However, check the feeding tables on any infant formula and it covers feeding from 0 to 12 months. Follow-on formula is not necessary.
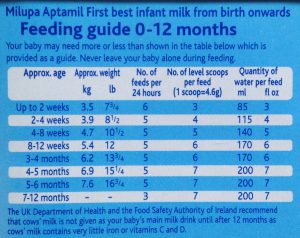
Companies make claims of supposed benefits of the nutrients added to follow-on formula and so-called “growing-up” milks, but these have no benefits over a normal healthy diet. Independent information on products and company claims is available. For example, see First Steps Nutrition Trust.
The World Health Organisation has made it clear that the prohibition on formula promotion covers follow-on formula and milks for older babies. UK legislation does not reflect this, despite the UN Committee on the Rights of the Child calling three times (in 2002, 2008 and 2016) for successive governments to bring legislation into line with the International Code of Marketing of Breastmilk Substitutes and subsequent, relevant Resolutions of the World Health Assembly.
The Code and Resolutions aim to protect breastfeeding AND to ensure “the proper use of breastmilk substitutes, when these are necessary, on the basis of adequate information and through appropriate marketing and distribution.”
Mike Brady, Campaigns and Networking Coordinator at Baby Milk Action, said:
“It is disappointing that a prestigious institution such as the Natural History Museum is hosting this event. Danone in misleading parents by undermining NHS advice to parents who use formula and breaking international marketing standards for these products. We are directing the public to NHS Choices, which explains that babies fed on formula do not need to progress onto follow-on formula and so-called growing-up milks. These are unnecessary products that rip off parents.
“We suggest the Museum put in place a policy on the events it will associate itself with. While it has told us it makes it clear to the third party host that agreeing to an event is not an endorsement of their product, service or views, the public are associating the promotion with the museum.
“Unfortunately the Natural History Museum is an ‘exempt charity’ and so outside the remit of the Charity Commission that has issued guidance in the past regarding the need for due diligence when considering links with commercial partners. We have contacted the responsible government department (the Department for Culture, Media and Sport) to ask it to intervene so that the public is not misled for the commercial gain of Danone and it issues its own guidance.”
Further details of the regulations and evidence of marketing practices used by Danone and other companies are contained in Baby Milk Action’s report Look What They’re Doing in the UK 2017.
Update 11:06 25 July. The Natural History Museum press office has informed Baby Milk Action:
“Aptamil is not a partner of the Museum and their involvement with the event is through Time Out, who is delivering the event. We are unable to comment on the background behind this partnership. As a world leading visitor attraction, breast feeding is fully encouraged throughout all of our public areas and events.”
Natural History Museum contact page.
For further information contact Mike Brady at info@babymilkaction.org
Notes for editors:
The Guidance Notes from the Department of Health on interpreting the Infant Formula and Follow-on Formula Regulations (2007) state:
48. In order to achieve compliance, companies will therefore need to [inter alia] ensure that formula advertising does not:
• promote a range of formula products by making the brand the focus of the advert, rather than specific products (e.g. where specific products are mentioned only in a footnote or in a picture of a tin of formula within the advertisement)
The advertising for this event (above) promotes the Aptamil logo and branding used for the infant formula (shown below) and so appears to be illegal. Accordingly, Baby Milk Action has asked Trading Standards to investigate.

