Baby Milk Action press release 29 October 2015
Media coverage: Huffington Post 5 November
The British Medical Journal (BMJ) announced yesterday that it has finally retracted a fraudulent study used by Nestlé, Mead Johnson and other formula companies to weaken laws all over the world in order to create a multi-million pound market for so-called hypoallergenic formulas. Claims for these products have been challenged since the 1980s. The work of Professor Ranjit Chandra came under scrutiny in 1994, when a nurse who worked for Chandra on his research in Canada revealed that published papers referred to far more mothers and babies than had been recruited to the studies. It has taken decades of campaigning by Baby Milk Action, editors and journalists, and other IBFAN groups, particularly Infact Canada (which questioned Prof. Chandra’s research from the beginning), for action to be taken.
Chandra’s reputation as a scientist started to unravel when he submitted a study to the British Medical Journal in Spring 2000 about the effects of his own patented multivitamin on the memories of seniors. BMJ Editor-in-chief at that time, Richard Smith, asked a statistician to look at it and was told that “the data had all the hallmarks of being entirely invented.” The BMJ rejected Dr. Chandra’s study and asked Memorial University where he was based to investigate.
In 2006 the Canadian Broadcasting Corporation (CBC) ran a three-part investigation into Professor Chandra (watch it at the bottom of this post). Chandra sued CBC and a jury ruled this summer that the allegations against him were “true”.
This week the BMJ finally retracted a Chandra study it published in 1989 based on research he conducted for Mead Johnson: “This concluded that mothers with a family history of allergy should use hypoallergenic (hydrolysed) formula feed if they were not breast feeding.”
Nestlé, Mead Johnson and other companies continue to promote hypoallergenic formulas. Nestlé’s “NEW SMA HA Infant Milk” is the focus of a new marketing campaign targeting health workers in the UK (below, Nestlé continues to claim that its SMA HA Infant Milk is “clinically proven to reduce risk of developing cow’s milk protein allergy”).
Nestlé has recruited a network of Clinical Representatives, with a job description giving their main responsibility as obtaining brand endorsements for SMA products from health professionals.
Mike Brady, Campaigns and Networking Coordinator at Baby Milk Action, said:
“This scandal demonstrates the importance of scrutinising company-backed research and seeking out independent information. Nestlé used Chandra’s discredited research for over a decade to build the marketing for so-called hypoallergenic formulas. As it embarks on another major marketing campaign for these milks, independent reviews cast serious doubts on the validity of the claims it makes today, with the Cochrane Library, Food and Drug Administration (FDA) and National Institute of Health and Care Excellence (NICE) all stating there is little or no evidence of any benefit from these formulas. But it is a multi-million pound market and Nestlé is today effectively encouraging parents to self-diagnose their children as being at risk of developing cows’ milk protein allergy, while it targets health workers with misleading information and study days.”
Nestlé is the target of a boycott because of its systematic violations of baby milk marketing rules. Baby Milk Action is currently promoting the boycott through International Nestlé-Free Week (26 October – 1 November).
Professor Chandra is now selling IMMUNOBOOST nutrition supplements in India as Chairman and Managing Director of Peridot Life Sciences.
For further information contact:
Mike Brady
Patti Rundall on 07786 523493 – prundall@babymilkaction.org
Supporting information
In an article posted on 28 October 2015, the BMJ writes:
[Chandra’s university] had already investigated Chandra for suspected data fabrication, prompted by his research nurse Marilyn Harvey, whom Chandra later tried to sue. She was responsible for recruitment to the infant formula studies Chandra had been asked to carry out for Ross Laboratories, Nestlé, and Mead Johnson in the 1980s.
She told internal university investigators in 1994 that the numbers of infants recruited and reported on for the Nestlé study didn’t match and that neither a three year follow-up study of these infants nor the Mead Johnson study had ever been carried out.
The Canadian Broadcasting Corporation (CBC) ran a three-part investigation into Chandra in 2006. Chandra unsuccessfully sued CBC, the jury in the case ruling this summer that the claims were “true”. (From the archive: Baby Milk Action press release from the time of the broadcasts).
In July 2004 Baby Milk Action reported Nestlé to the Advertising Standards Authority when it tried to  launch NAN HA in the UK (right, a stand targeting health workers that year). The ASA refused to investigate on the grounds that the publishers and health workers should be able to judge whether claims were correct or not.
launch NAN HA in the UK (right, a stand targeting health workers that year). The ASA refused to investigate on the grounds that the publishers and health workers should be able to judge whether claims were correct or not.
Nestlé referenced Chandra in claiming: “Nan HA significantly reduces the potential for atopic symptoms (eczema, asthma, rhinitis) in infants with a strong family history of allergy” and “Nan HA is palatable and affordable”.
In a brochure distributed to the public on a Nestlé stand at an Allergy UK event in 2004, Nestlé referenced Chandra in claiming:
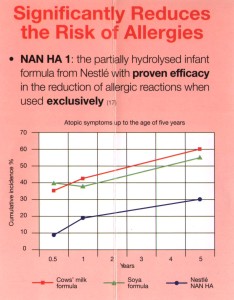 “Significantly Reduces the Risk of Allergies. Nan HA 1: the partially hydrolysed infant formula from Nestlé with proven efficacy in the reduction of allergic reactions when used exclusively.” [stress as in the original – left].
“Significantly Reduces the Risk of Allergies. Nan HA 1: the partially hydrolysed infant formula from Nestlé with proven efficacy in the reduction of allergic reactions when used exclusively.” [stress as in the original – left].
In the US Nestlé used Chandra’s research to promote its Carnation “Good Start” formula.
At one time it made a hypoallergenic claim for this formula, but removed it after legal action was brought by 9 US States over babies suffering anaphylactic shock after being fed on it. Attorney General, Robert Adams, said in 1989: “In its eagerness to break into the lucrative US formula market [Nestlé] Carnation led the public to believe that it had created a formula that could not cause an allergic reaction.” Adding, “Those babies who had severe allergic reactions to Carnation Good Start have paid a high price for the company’s irresponsible conduct.” (original press report).
In its article retracting the study, the BMJ states:
Nestlé declined to respond directly to The BMJ’s questions about the thoroughness of its monitoring of the Carnation study or whether it had been under pressure in 1988 to provide evidence to the US regulator of the benefits of its infant formula, as CBC had claimed.
But in a statement Catherine O’Brien, vice president of corporate affairs at Nestlé Canada, said that the company had cooperated fully with Memorial’s formal investigation and had ceased referencing Chandra’s study in 2006.
While Nestlé references other studies, independent reviews do not substantiate the hypoallergenic claims either. The charity First Steps Nutrition Trust critically appraises studies cited by companies and has produced a briefing on Partially hydrolysed whey based infant formula and the prevention of allergy: A summary of current evidence and policy.
This quotes the an independent, systematic review of the evidence from the Cochrane Library as follows:
“There is no evidence to support feeding with a hydrolysed formula to prevent allergy in preference to exclusive breastfeeding. In infants at high risk for allergy who are unable to be completely breastfed, there is limited evidence that feeding with a hydrolysed formula compared to a cow’s milk formula reduces allergies in babies and children, including cow’s milk allergy. Concerns regarding quality of the evidence and consistency of the results indicates further studies are needed”
In the UK, public health guidance from the National Institute of Health and Care Excellence (NICE) concluded from an extensive literature review that:
“There is insufficient evidence that infant formulas based on partially or extensively hydrolysed cows’ milk protein can prevent allergies” (NICE PHG11, 2008),
First Steps Nutrition points out there are risks from using hypoallergenic formulas with allergic children. The US Food and Drugs Administration requires warnings stating:
“Partially hydrolysed formulas should not be fed to infants who are allergic to milk or to infants with existing milk allergy symptoms. If you suspect your baby is already allergic to milk, or if your baby is on a special formula for the treatment of allergy, your baby’s care and feeding choices should be under a doctor’s supervision.”
Despite this, in the UK, Nestlé SMA HA is sold through retailers such as Boots with text that effectively encourages parents to self-diagnose their child’s risk of developing cow’s milk allergy.
Nestlé is prohibited from targeting health workers directly by most health facilities in the UK; under UNICEF Baby Friendly guidelines companies can only provide scientific and factual information to a designated expert for assessment. Nestlé attempts to bypass this restriction by organising its own events and inviting health workers to those, with the offer of refreshments and talks on subjects that may be of interest. Several events are planned in November. It is striking that an event on the “Unsettled Baby” involves two hours of product promotion, with only 15 minutes on the headline subject, which is presented by a Nestlé representative in any case.
Such events break World Health Assembly marketing requirements. Baby Milk Action encourages health workers to say NO to formula company sponsorship and study days and will be organising protests at these events. Click here for further information.
To help Baby Milk Action in its work:




Pingback: British Medical Journal Announces Hypoallergenic Baby Milk Research "Fraudulent", Retracts Paper - Herbs Info